URGENT UPDATE: A groundbreaking study from a collaborative team of scientists at Jiangsu University, the Chinese Academy of Sciences, Hasselt University, and MIT reveals a revolutionary approach to sustainable energy production through integrated electrosynthesis. Published online in eScience in July 2025, this research addresses the pressing need for innovative solutions to reduce carbon emissions and enhance energy efficiency.
For over two centuries, fossil fuels have dominated energy and chemical production, leading to over 80% of global consumption and a significant rise in CO2 emissions. This reliance has exacerbated climate change and environmental degradation. With renewable energy investments on the rise, traditional chemical processes remain carbon-intensive and economically inflexible. The latest findings highlight a promising pathway: electrochemistry powered by renewable energy, capable of transforming chemical manufacturing.
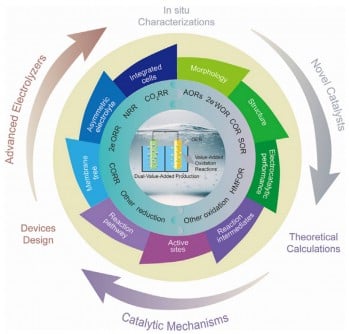
In their comprehensive review, the researchers emphasize the urgent need to replace the inefficient oxygen evolution reaction (OER) with alternative oxidation reactions. By integrating these reactions with reduction processes, such as CO2 and nitrogen conversion, the study outlines how to achieve dual-value outputs—clean fuels and market-relevant chemicals—while dramatically improving system efficiency.
The research team details critical advancements in catalysts, electrolyzers, and reaction mechanisms that enable this innovative coupling. By utilizing alternative oxidation reactions—like methanol and glycerol oxidation—researchers report significant improvements in system performance, yielding valuable by-products such as formic acid, acetic acid, and hydrogen peroxide.
Prof. Zhenhai Wen, one of the lead authors, stated,
“Electrochemical systems that simultaneously produce two valuable outputs represent a paradigm shift for green chemistry.”
This approach not only lowers energy barriers but also supports the production of high-value chemicals alongside clean fuels, marking a crucial step towards a sustainable circular chemical industry.
The study highlights advancements in nanostructured materials and novel electrolyzer architectures that enhance stability and conversion rates. Hybrid electrolyzers are evolving from traditional H-type cells to flow cells, enabling industrial-scale current densities. These developments are complemented by advanced in situ and operando techniques, allowing researchers to monitor catalytic processes in real-time, thus accelerating catalyst design and optimization.
The implications of these findings are profound. The development of dual-value electrosynthesis systems holds significant promise for addressing climate and resource challenges. Beyond reducing carbon emissions, these systems facilitate the cost-effective production of green hydrogen, fuels, fertilizers, and chemical feedstocks.
As global net-zero ambitions grow, this research presents a clear roadmap for transforming chemical manufacturing into a low-carbon process. The integration of advanced catalysts, computational methods, and industrial-scale electrolyzers could revolutionize the landscape of renewable-driven industrial chemistry.
What happens next? Researchers and industry leaders will be watching closely as this innovative approach to electrosynthesis gains traction. With the potential to reshape energy and chemical industries, this development is crucial for fostering a sustainable future.
For more details, access the full study at DOI: 10.1016/j.esci.2024.100333. This groundbreaking research was supported by the National Natural Science Foundation of China and other key funding bodies, underscoring its importance in the global push for sustainable energy solutions.
Stay tuned for more updates as the developments in electrosynthesis evolve, promising a cleaner, greener future for all.