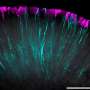
Research teams at the University of California, San Diego and the Research Institute of Molecular Medicine have successfully engineered vascularized retinal organoids that incorporate functional light-signal pathways. This groundbreaking advancement addresses a significant challenge in regenerative medicine, particularly concerning the maintenance of retinal ganglion cells, which play a crucial role in vision.
Retinal organoids, which are miniature, simplified versions of the retina, have long struggled to sustain the health of retinal ganglion cells over prolonged periods. Traditionally, the densely packed tissues in these organoids limit the supply of vital nutrients and oxygen, leading to cell death. The inability to maintain these cells hampers the potential for research into retinal diseases and therapies.
The recent work, published in September 2023, demonstrates a novel approach to overcoming this limitation. By incorporating vascularization into the organoid structure, researchers significantly improved nutrient and oxygen delivery to the cells. This innovation not only enhances cell survival rates but also opens new pathways for studying retinal diseases in a lab setting.
Breakthrough in Retinal Research
The development of these vascularized retinal organoids is a pivotal step forward in the field of ophthalmology and regenerative medicine. The ability to maintain healthy retinal ganglion cells for extended periods allows researchers to conduct more in-depth studies of retinal diseases such as glaucoma and age-related macular degeneration.
According to the research team, this advancement enables a more accurate representation of the human retina, which is essential for testing potential therapies and understanding disease mechanisms. The integration of functional light-signal pathways within the organoids mimics the natural environment of the retina, further enhancing the relevance of the research findings.
This breakthrough not only benefits scientific research but also has potential implications for developing patient-specific treatments. By utilizing a patient’s own cells to create organoids, personalized therapies could be designed to combat specific retinal conditions.
Future Implications for Vision Restoration
The implications of this research extend beyond basic science. The ability to sustain retinal ganglion cells may lead to advancements in regenerative therapies aimed at restoring vision. As scientists continue to refine these organoids, the prospect of developing effective treatments for previously untreatable retinal diseases becomes increasingly feasible.
Moreover, the funding support from the National Institutes of Health underscores the significance of this research in addressing public health concerns related to vision loss. As the global population ages, the demand for effective treatments for age-related retinal conditions will likely increase.
In summary, the creation of vascularized retinal organoids with functional light-signal pathways marks a significant milestone in retinal research. This innovation paves the way for improved understanding of retinal diseases and has the potential to transform therapeutic approaches in the field of ophthalmology. As researchers continue to explore the capabilities of these organoids, the future of vision restoration looks promising.