Profusa, Inc. announced that its LumeeTM Oxygen tissue monitoring technology successfully met primary end points in a recent clinical study focused on patients with peripheral artery disease (PAD). The results were presented during the late-breaking clinical trials session at the Paris Vascular Insights (PVI) 2025 conference on December 13, 2025. This promising data supports the potential submission to the U.S. Food and Drug Administration (FDA).
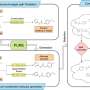
The study, conducted in collaboration with the University of California San Francisco, the San Francisco VA Medical Center, and San Francisco General Hospital, demonstrated that Lumee oxygen tissue monitoring is a safe and effective long-term solution for managing PAD. The findings also showed a strong correlation between Lumee’s readings and traditional measurements of oxygen levels, specifically the transcutaneous partial pressure of oxygen (tcpO2).
Key Findings from the Clinical Study
The presentation, titled “Monitoring Tissue Oxygen Dynamics with a Novel Implantable Hydrogel Sensor in Patients with Peripheral Arterial Disease,” highlighted data from 15 PAD patients who had subcutaneous hydrogel sensors implanted in their arms and feet. These sensors monitored tissue oxygenation over a one-year period, with assessments at multiple intervals, including days 2, 10, 90, 180, and 365.
Ben Hwang, Ph.D., Chairman and CEO of Profusa, expressed optimism about the findings. He stated, “Our Lumee oxygen monitoring technology was designed for use both in the clinic and at home. We are pleased by the presented data, which will support our potential U.S. FDA submission and our goal of making long-term oxygen tissue monitoring easily accessible at home to improve overall patient outcomes.”
The study results indicate that Profusa is on track to begin commercializing Lumee tissue oxygen monitoring in the European Union in the second quarter of 2026. With over 716,000 annual critical limb ischemia procedures performed in Europe, the company aims to meet the needs of this significant market.
About Profusa and Its Technology
Based in Berkeley, California, Profusa is a commercial stage digital health company that has developed a next-generation technology platform for continuous biochemical monitoring. The company’s innovative biosensors are designed to deliver actionable, medical-grade data for both personal and clinical use. By providing a personalized biochemical signature, Profusa seeks to empower clinicians and patients alike with reliable health information.
The Lumee technology is one of several initiatives by Profusa to transform health monitoring. The company’s commitment to developing long-lasting, injectable, and affordable biosensors reflects its mission to improve patient care through advanced technology.
For more information about Profusa and its innovations, visit https://profusa.com.
As the company moves forward with plans for the FDA submission and European commercialization, it faces the usual risks and uncertainties associated with forward-looking statements in the health sector. Investors are encouraged to consider these factors when evaluating Profusa’s prospects.
For media inquiries, contact Profusa’s investor relations at [email protected] or call 1 (212) 655-0924.