Researchers at Northwestern University have developed a groundbreaking device designed to monitor the vital signs of fetuses during surgical procedures. This innovative collaboration with the Ann & Robert H. Lurie Children’s Hospital in Chicago was detailed in a study published on January 26, 2023, in the journal Nature Biomedical Engineering.
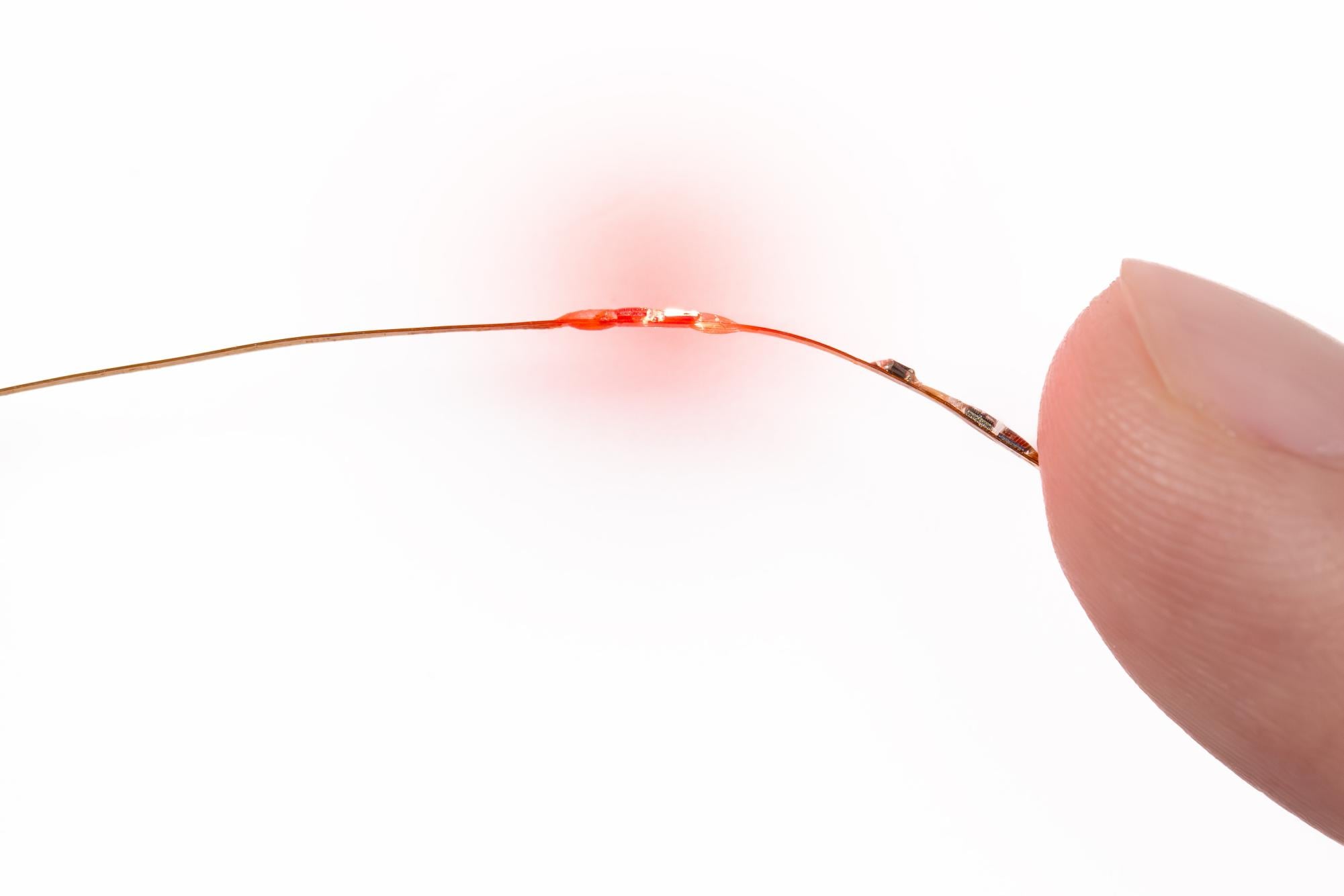
The newly created device is remarkably thin, akin to a human hair, and is capable of measuring vital signs such as heart rate, temperature, blood oxygen levels, and heart rate variability. It functions through small ports in the body that are typically created for fetal surgery. John Rogers, the director of the Querrey Simpson Institute for Bioelectronics at Northwestern and the study’s lead author, emphasized the significance of this advancement. Currently, there is no method for continuously monitoring these vital signs in fetuses, which contrasts with the established practices for monitoring infants and adults during surgery.
Tracking a patient’s vital signs in real time is crucial for ensuring their safety during surgical procedures. “You’d like to be able to track the patient’s status throughout the surgery, so that you could detect any kind of adverse effect as soon as possible,” Rogers explained. The device employs “extremely miniaturized” sensors, and its design includes small balloons that facilitate maneuverability through the ports while maintaining contact with the fetus.
The need for such a device arises from the limitations of current monitoring techniques. Steven Papastefan, a general surgery resident at Northwestern, noted that existing methods, such as transuterine echocardiography, can only assess the fetus’s heart rate every ten minutes during surgery. This sporadic monitoring can lead to uncertainty about the fetus’s condition. “We check its heart rate to make sure it’s okay, and then we keep operating for another ten minutes,” Papastefan said. “With this device, we can continuously monitor the fetus throughout the operation.”
Fetal surgeries, while infrequent, can be essential in preventing irreversible damage as the baby develops. Conditions such as twin-twin transfusion syndrome and spina bifida often necessitate surgical intervention before birth. Aimen Shaaban, director of the Chicago Institute for Fetal Health at Lurie Children’s Hospital and a co-author of the study, explained the implications of spina bifida, where an opening in the spine can lead to severe neurological issues. Closing this opening before birth can significantly alleviate complications and improve developmental outcomes.
Shaaban described the collaborative effort in developing the device as “good old-fashioned collaboration” between the teams at Northwestern and Lurie Children’s Hospital. Testing the device involved surgical procedures on lambs, where researchers created spina bifida in lamb fetuses to evaluate the device’s effectiveness during repair operations. Although the device has yet to be tested in humans, Rogers estimates that human trials could begin within two to three years, following the necessary regulatory approvals.
At Lurie Children’s Hospital, fetal surgeries occur once or twice a week. Shaaban expressed optimism about the device’s potential to transform surgical practices. “It’s really going to change the way we actually care for the patients and give us greater insight into what we’re actually doing,” he said. “We think we do a good job, but with the data in front of us, we’ll know and be able to do a better job.”
This significant advancement in fetal monitoring technology underscores the ongoing commitment to improving surgical outcomes for vulnerable patients and highlights the innovative research emerging from collaborations in the medical field.