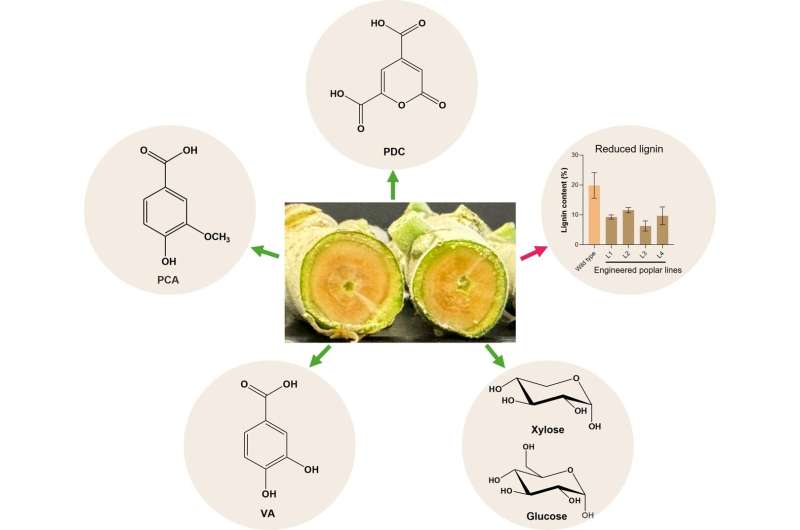
Researchers at Florida State University have made significant strides in understanding why certain light-driven chemical reactions often lose energy before achieving bond-breaking. Their findings, published in August 2023, reveal a specific pathway within certain molecules that redirects light energy, thereby limiting the efficiency of these reactions.
This groundbreaking study could have far-reaching implications for various industries, particularly in the development of pharmaceuticals and other chemical products. By addressing the energy loss that occurs during these reactions, scientists aim to enhance the efficiency of processes that rely on light energy, making them more viable for practical applications.
Unveiling the Mechanism
The research team focused their attention on a class of molecules known for their potential in catalyzing light-driven reactions. They identified a mechanism that diverts energy away from the bond-breaking process, which is crucial for the desired chemical transformations. Understanding this diversion is essential for scientists who seek to optimize these reactions.
The implications of this research extend beyond theoretical knowledge. With improved efficiency in chemical reactions, manufacturers could significantly lower production costs and reduce waste, particularly in the pharmaceutical sector. This could lead to more sustainable practices and more effective drugs reaching the market faster.
Future Applications
The insights gained from this study are expected to pave the way for the creation of new catalysts that harness light energy more effectively. As the demand for sustainable and efficient chemical processes grows, researchers are optimistic that their work will contribute to advancements in green chemistry.
The findings from Florida State University highlight the importance of interdisciplinary research in tackling complex scientific challenges. By bridging the gap between chemistry and energy research, this study opens new doors for innovations that could benefit society at large.
In conclusion, the study not only sheds light on the fundamental aspects of chemical reactions but also holds promise for enhancing the efficiency of light-driven processes. As researchers continue to explore these pathways, the potential for practical applications in pharmaceuticals and beyond remains vast and exciting.