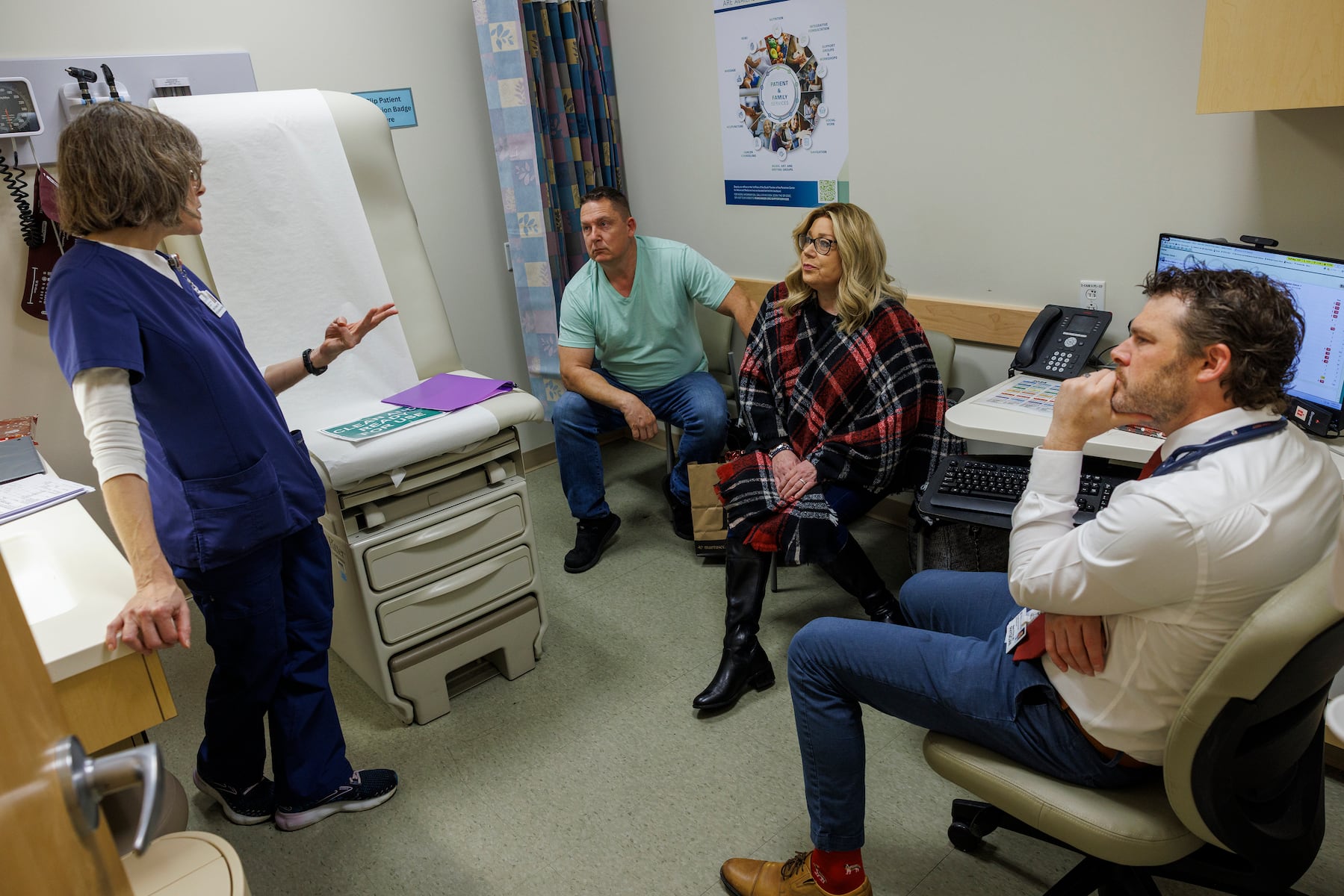
PHILADELPHIA — A new drug called daraxonrasib is showing promise for patients battling pancreatic cancer, particularly for those in late-stage diagnoses. Irene Blair, a 59-year-old grandmother from Newark, Delaware, was given a prognosis of six to eight months to live in June after her cancer advanced to stage 4. Participating in clinical trials at Penn Medicine’s Abramson Cancer Center, Blair’s best hope lies in this innovative treatment, which aims to prolong survival for a disease with a dismal five-year survival rate of just 13%.
The drug, a KRAS inhibitor, targets a protein known for driving the growth of pancreatic cancer, classified as one of the most aggressive types of cancer. With recent trial results indicating a significant advancement in treatment options, the drug is garnering attention from researchers and patients alike. The U.S. government has expedited its review process following early results from the clinical trials conducted by Revolution Medicines, Inc., based in California.
In a phase 1 trial, involving 38 patients, daraxonrasib doubled the average survival time from standard chemotherapy, increasing it from approximately 7 months to over 15.6 months for at least half of the participants. Mark O’Hara, an oncologist at Penn and leader of multiple KRAS inhibitor trials, emphasized the need for effective treatments beyond chemotherapy, which has limited efficacy for pancreatic cancer patients.
Blair commenced treatment through a phase 3 trial in July. Remarkably, within three weeks, her cancer-related pain subsided. Scans in October revealed stable or shrinking tumors, and her most recent scan in December confirmed that her cancer had not progressed. The therapy has allowed her to reclaim a sense of normalcy, a stark contrast to her experience on chemotherapy, which caused her to lose 35 pounds and left her too weak to walk.
Blair is now eager to make the most of her time. Having retired from real estate in May, she plans to travel to visit family in California and Florida. Reflecting on her journey, she expressed the emotional toll of living with cancer, stating, “You just wonder, ‘Will I be here next year?’”
The quest to develop a drug that effectively targets the KRAS protein began with its discovery in 1982. The mutated KRAS protein acts like a “gas pedal” for cancer proliferation, contributing to the aggressiveness of various cancers, particularly pancreatic, lung, and colon cancers. The breakthrough in developing KRAS inhibitors came in 2021, when the FDA approved the first drugs targeting this protein for lung cancer. Since then, numerous KRAS inhibitors have entered various stages of development, with daraxonrasib being one of the first to undergo trials specifically for pancreatic cancer, where nearly 90% of cases exhibit these mutations.
As a “pan-RAS inhibitor,” daraxonrasib not only targets KRAS but also affects two other related proteins, HRAS and NRAS, that can drive cancer when mutated. In phase 1 trials, more than 90% of the 83 patients experienced stabilization of their cancer, with approximately 30% showing shrinkage. For many participants, the drug extended the period before cancer progression by more than eight months.
The treatment regimen consists of taking three pills daily at home. The most common side effect reported is a rash, which occurred in 91% of patients during the trial, with 8% experiencing severe cases. Other side effects include diarrhea, nausea, and mouth sores. O’Hara indicated that these symptoms are generally manageable and allow for a better quality of life compared to traditional chemotherapy.
“I want to be able to give KRAS inhibitors to all my patients right now,” O’Hara remarked, highlighting the urgency and hope surrounding this new treatment option. As clinical trials progress, the medical community remains optimistic that daraxonrasib could transform the landscape of pancreatic cancer treatment, providing patients like Irene Blair with the precious gift of time.