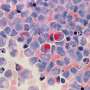
A research team at Oregon Health & Science University has unveiled a promising drug combination that could significantly enhance treatment for patients with acute myeloid leukemia (AML) facing resistance to standard therapies. This breakthrough was detailed in a study published in Cell Reports Medicine, where researchers explored over 300 patient samples. Their findings reveal that combining venetoclax, a standard AML treatment, with palbociclib, a cell-cycle inhibitor approved for breast cancer, delivers markedly improved and lasting anti-leukemia effects compared to venetoclax alone.
The research was led by Melissa Stewart, Ph.D., who expressed excitement about the results. “Of the 25 drug combinations tested, venetoclax plus palbociclib was the most effective. That really motivated us to dig deeper into why it works so well—and why it appears to overcome resistance seen with current therapy,” she explained. This combination therapy shows great promise as it addresses a significant hurdle in AML treatment—drug resistance.
Understanding Drug Resistance in AML
Every year, over 20,000 Americans receive an AML diagnosis, making it one of the most prevalent and aggressive types of leukemia. Despite advancements in treatment, drug resistance remains a pervasive challenge. Since its approval by the Food and Drug Administration in 2019, venetoclax combined with azacitidine has become a preferred treatment option. Yet, as Jeffrey Tyner, Ph.D., a professor at OHSU and corresponding author of the study, notes, “Unfortunately, almost everyone will eventually have drug resistance.”
Current treatments improve initial response rates and quality of life, but the five-year survival rate for AML is still around 25% to 40%. Tyner, a co-leader of the national Beat AML 1.0 program, emphasized the significance of this new research, stating, “This combination was nominated from the Beat AML data, and Dr. Stewart validated that prediction, showing not only that it works, but why.”
In the study, AML cells exposed to venetoclax alone attempted to adapt by increasing protein production, allowing them to survive. The addition of palbociclib inhibited this adaptation, effectively regulating the protein-production mechanisms within the cells. “Patient samples that responded strongly to the combination showed clear downregulation of genes involved in protein synthesis,” Stewart reported, highlighting a critical discovery.
Promising Results from Animal Models
The research team also conducted tests using mouse models implanted with human AML cells known to carry mutations that cause venetoclax resistance. Results indicated that venetoclax alone did not extend survival, consistent with genetic expectations. In contrast, the combination treatment resulted in a majority of the mice surviving for 11 to 12 months, with one mouse still alive at the conclusion of the study.
Stewart shared a personal connection to the research, stating, “I’m a breast cancer survivor and was treated here at OHSU, so I know what it’s like to be a cancer patient. The hope that research and clinical trials can bring—that’s what motivates me. Working on AML gave me a way to contribute.”
Tyner and Stewart underscored the importance of following scientific data beyond conventional boundaries. Tyner noted, “Some might ask why a breast cancer drug would work in AML. But biology can be shared across very different cancers. This is a great example of why keeping an open mind matters and following the data where it leads.”
Looking ahead, the research team is already assessing other drugs similar to palbociclib, many of which are also approved for breast cancer, to broaden future clinical trial options. Tyner expressed optimism about the next steps, stating, “We haven’t tested it in patients yet, but based on everything we’ve seen, our prediction is that this combination would mitigate most known resistance mechanisms to the current standard therapy. Making it a clinical reality will take work, but this is exactly why we do what we do.”
The study, titled “CDK4/6 inhibition overcomes venetoclax resistance mechanisms with enhanced combination activity in Acute Myeloid Leukemia,” appears in Cell Reports Medicine.