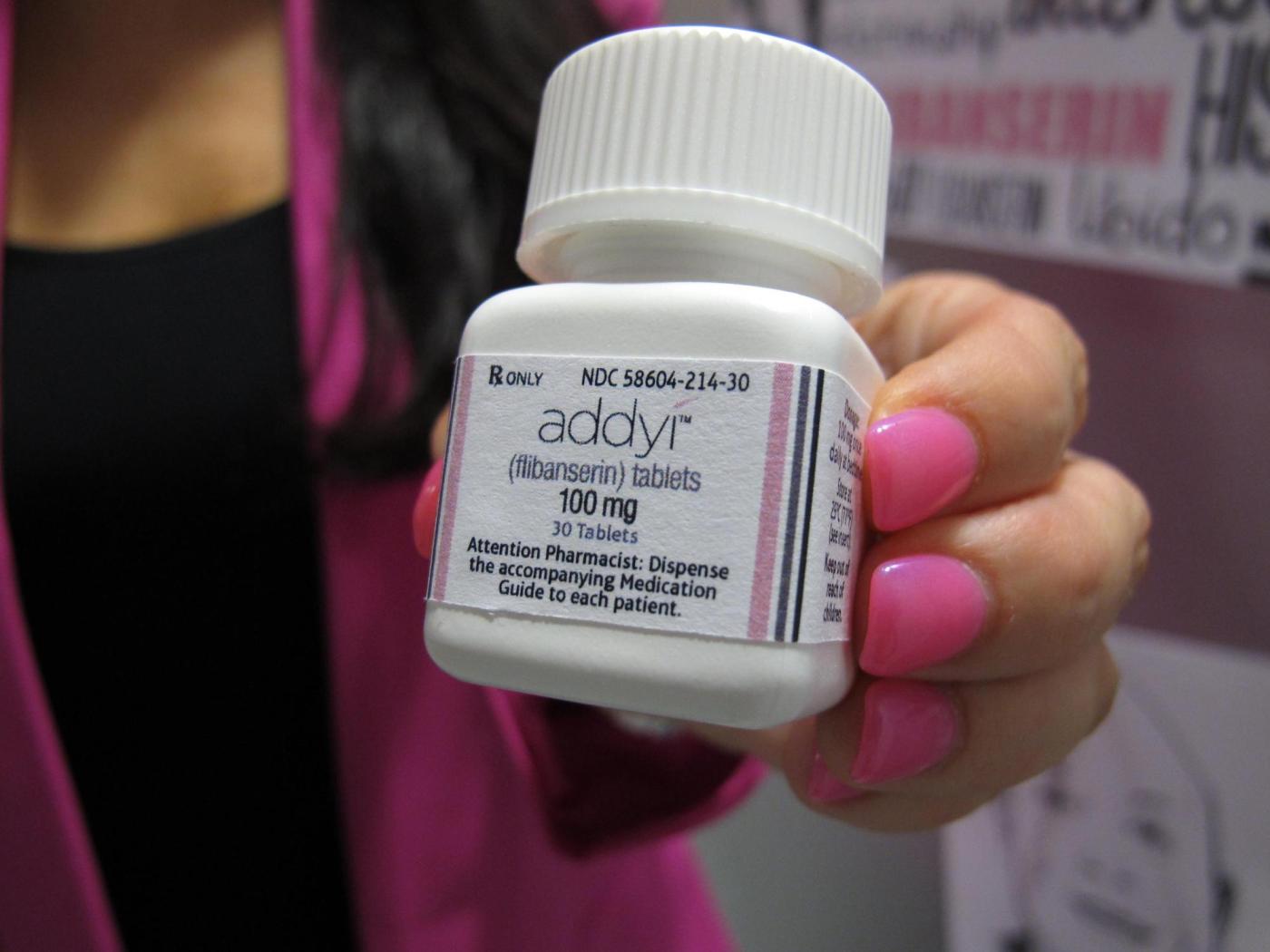
The U.S. Food and Drug Administration (FDA) has expanded the approval of the libido-enhancing drug Addyi, allowing women over the age of 65 who have gone through menopause to use the medication. This announcement, made on March 25, 2024, marks a significant shift in the drug’s accessibility, broadening its use beyond the originally approved demographic of premenopausal women.
Addyi, produced by Sprout Pharmaceuticals, was first approved in 2015 after a controversial journey through the regulatory process. Initially, the drug was designed to address hypoactive sexual desire disorder, a condition characterized by a persistently low sexual appetite that affects many women. According to surveys, this condition is believed to impact a notable portion of American women.
While Addyi was anticipated to fill an important gap in women’s health, its journey has been complicated. The drug can produce side effects such as dizziness and nausea, and it carries a serious safety warning regarding alcohol consumption. The FDA has classified this warning as a boxed warning, its most severe designation. This label indicates that mixing Addyi with alcohol could result in dangerously low blood pressure and fainting.
Sales of Addyi have not met initial expectations. In 2019, the FDA approved a second option for low female libido—a non-invasive injection that works through a different neurological pathway. Despite these challenges, Sprout Pharmaceuticals remains optimistic. CEO Cindy Eckert stated that the recent approval reflects a decade of advocacy aimed at changing perceptions around women’s sexual health.
Diagnosing hypoactive sexual desire disorder is complex. A range of factors can influence libido, particularly after menopause, when hormonal changes can lead to various biological and psychological symptoms. Physicians are tasked with ruling out other potential causes, such as relationship issues, medical conditions, and mental health disorders, before considering medication.
The recognition of hypoactive sexual desire disorder as a medical condition dates back to the 1990s. This was also a period when the success of Viagra for men prompted increased investment in research for female sexual dysfunction. However, the diagnosis remains contentious, with some psychologists challenging the notion that low libido should be classified as a medical issue.
Prior to its approval, Addyi faced rejection from the FDA on two occasions due to concerns over its modest effectiveness and associated side effects. The eventual approval followed significant lobbying efforts by the company and advocacy groups like Even the Score, which framed the lack of treatment options for women as a matter of women’s rights.
As the FDA moves towards a more inclusive understanding of women’s health, the approval of Addyi for older women signifies a step forward in addressing the complexities of female sexual wellness. The ongoing dialogue about women’s health issues continues to evolve, reflecting broader societal changes in the understanding of sexual health and wellness.